
How to spot a fake KN95 respirator mask from China.
SOURCE: https://www.cdc.gov/niosh/npptl/usernotices/counterfeitResp.html
How to spot a fake KN95 respirator mask from China.

Over the past 3 weeks, I’ve been working with Bstrong and GEM to help authenticate the quality of the KN95 (N95 equivalent from China) respirators. I appreciate everyone’s heart and effort during this crisis by providing us with their export distributors in China. HOWEVER, I am finding that over 90% of the products being offered to us are NOT real KN95 respirators. Since many of you do not know about the FDA regulations and why medical providers need high-quality N95 respirators, please let me guide you.
Outline:
1. Why high-quality KN95 is important
2. Difference between FDA registration and FDA approval.
3. The FDA logo on the box is probably a low-quality KN95.
4. Spot FAKE Chinese KN95/NIOSH knockoffs.
5. Lower-quality KN95 has ear loops.
6. N95 is equal to FFP2 and “real” KN95
7. You might kill a medical provider by giving them a bad respirator
8. 4 easy ways to help spot a fake

Low vs High quality KN95
Imagine that your face is a bank vault, the mask is a bank vault door and the virus is a thief. The door should be as thick as possible to reduce the possibility of a thief going through the door (N95 means 95% filtration). However, the SEAL of the door on the door frame is equally important. If there is a large gap between the vault door and the vault door frame, then the thief can easily get inside the bank vault.
Most suppliers don’t know this, but 3M respirators that are FDA-cleared have different sizes. It is NOT a one size fits all. Furthermore, all hospital employees undergo fit testing to ensure a tight seal. The majority of the low-quality KN95 has a loose seal. While these low-quality respirators may have proof of testing of KN95 grade quality in China, this is the test of just the respirator (the vault door) and NOT the seal (door frame). We have already imported several of these low-quality respirators and have found that the fit is poor and the elastic is too loose to pass a hospital fit testing. Many of us using N95 respirators are face to face with coughing or vomiting patients. We want the quality respirators with the tightest seal and not any respirator that will let the thief into the bank vault. This is why many health care workers have a mask-shaped bruise or line on their faces.


FDA registration vs. approval
The phrase I hear from suppliers is that their KN95 is FDA approved (RED-FLAG).
1. FDA doesn’t approve N95 respirators. It is a class 2 device that only requires FDA clearance. Even 3M, the official N95 for hospitals, does not use the word approval, but FDA-cleared.
2. On March 28th, the FDA issued an Emergency Use Authorization. This authorization allowed the manufacturer to seek a EUA to market in the US. However, not ALL KN95 were authorized for use. Here is the list of KN95 that are Authorized Imported, Non-NIOSH Approved Respirators Manufactured in China as per the FDA.
3. If you import fraudulent masks from China and mislead a hospital, then you are NOT immune from liability. The HHS issued a declaration to provide liability immunity for claims against the distribution of PPE. However, it does not cover you from claims involving “willful misconduct.” Knowingly distributing fraudulent KN95 probably puts you in the “willful misconduct” category. Therefore, you have been warned.
4. Your FDA certificate is likely an FDA registration certificate and not an FDA-approval. FDA has a TWO step process where ANY company can REGISTER their product with the FDA before getting clearance or approval. This registration certificate is given to a company that paid the FDA fee to get registered. The registration certificate states the following:
“Registration of a device establishment or assignment of a registration number does not in any way denote approval of the establishment or its products. Any representation that creates an impression of official approval because of registration or possession of a registration number is misleading and constitutes misbranding.”
I find that most companies will try to mislead me to believe that their KN-95 is FDA-approved by submitting an FDA registration certificate as proof. Once again, FDA registration is NOT FDA approval.


FDA logo on the box
FDA does NOT allow a vendor to put the FDA logo on the box of a respirator. So, if your Chinese KN95 respirator has an FDA logo on the box, then you are probably importing a non-authorized KN95 respirator. This is the easiest way to help spot a suspicious KN95.


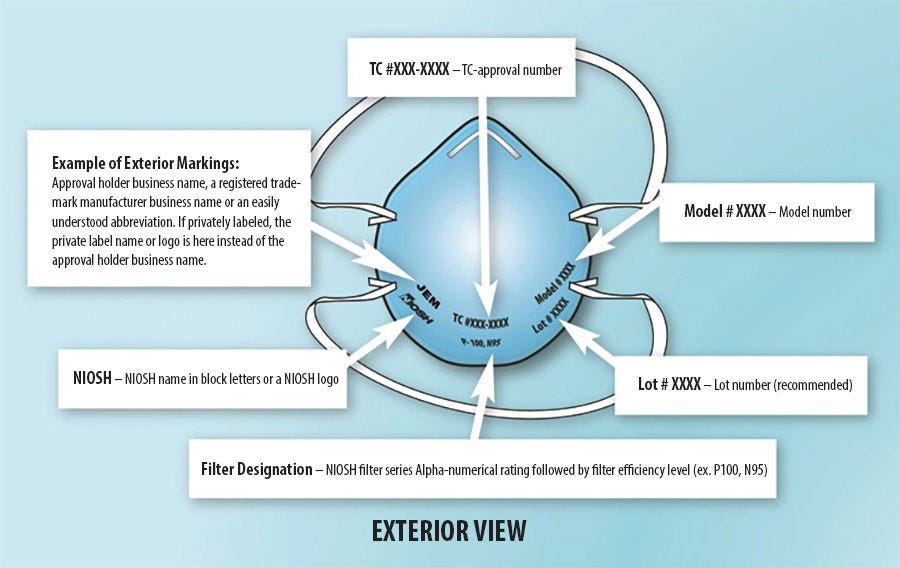
Counterfeit Chinese NIOSH respirators
The Chinese are notorious for exporting counterfeit goods. Unfortunately, it is no different when it comes to NIOSH(NIOSH is similar to N95). There are REAL Chinese NIOSH respirators being copied into counterfeits. Even the CDC has set up a webpage showing the difference between real and fake NIOSH respirators from China. If your respirator is on this list, then it’s fake and you are a participant in distributing counterfeit products. The CDC has also set up a webpage with the list of its NIOSH-approved respirators. Europe has also set up a similar website.
Low-quality KN95 have ear loops
These are examples of the FDA-cleared N95 respirators from 3M.
Do you notice the elastic bands? They all WRAP COMPLETELY around the head!


Here are the low-quality KN95 respirators.
Do you notice the elastic bands? They all LOOP around the ears!

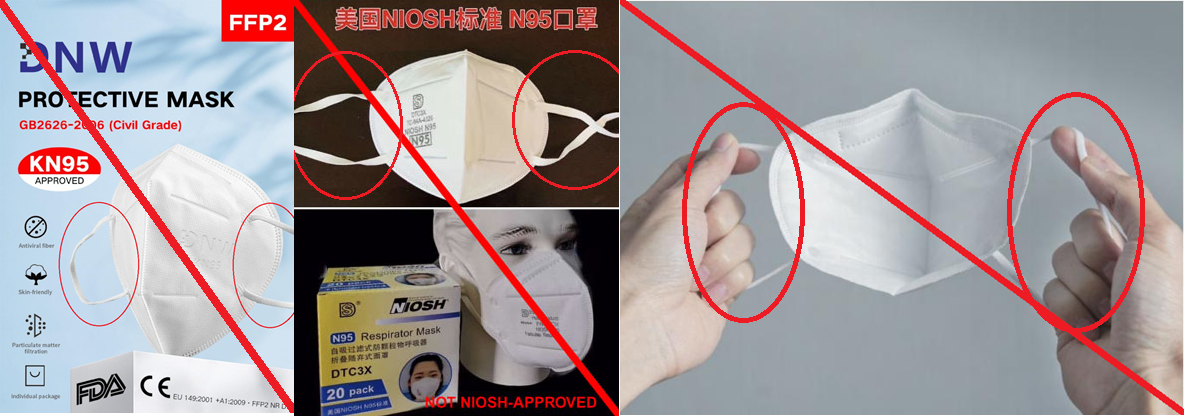
The difference in the elastic is in the analogy about the bank vault door and the door frame seal. We’ve tested many of these “ear loop” respirators and they are NOT tight enough. People have different ear sizes and distances from the face to the ear. While the quality of the respirator material may provide 95% filtration, the seal is inadequate to pass the fit testing on most medical providers. Most KN95 ear loop respirators are just one size that probably fits the face of an average Chinese person, but doesn’t account for the variety of head and face structures found in America. It should be noted the Dasheng does make official NIOSH masks, but the real Dasheng respirator’s elastic wraps completely around the head.

Know your N95 equivalent
We recently received a shipment of official European 3M FFP1 respirators from a vendor who misleadingly said that they were N95. Different countries will use different labeling to indicate similar functions. FFP1 respirators filter up to 80%, therefore they are NOT N95 equivalent. The closest European equivalent of N95 is FFP2 & P2 grade respirators, which are rated at 94%.
A heart without knowledge is foolish
I truly believe and hope that many of us are stepping up to the call and providing our front line medical staff with proper PPE. I understand the dire need as some of us are using garbage bags as additional PPE. But please understand that while something might be better than nothing, the way we interact with patients while using an N95 respirator is different than a simple surgical mask. Your importing of fake or lower quality respirators may cause one of my colleagues to die from the lack of proper protection. So, if you already imported fake KN95 or NIOSH, then tell the hospitals and providers about what they actually are. They can probably be used in non-high risk settings. But, if you withhold this knowledge, then you are probably engaging in “willful misconduct.”
Conclusion: How to spot a counterfeit or low-grade KN95 respirator mask.
FDA logo on the box
FDA registration certificate as proof of “FDA approval”
Earloops
Loosely packed masks without any packaging or labeling
On behalf of the medical providers, we thank you, the suppliers, for your heart.
Sincerely,
Jay Park MD
P.S. Shall we still buy low-quality respirators? If our hospitals are running out of PPE and they cannot source it from an authorized vendor, then we are left without a choice. Also, I believe that these low-quality KN95 respirators might be used in NON-CLINICAL settings (Recently, the Chinese government told European countries to “double check instructions.” On one of the boxes shown above, it states “Civil Grade.”) However, we, as suppliers and distributors, still have an obligation to be transparent about our products.
P.P.S. Want more on COVID-19?
KN95 Part 2: How to identify suspicious, fake or misleading marks & certificates with your KN95 respirator mask.
Alternative Medicine Treatment for COVID-19? Zinc and Green tea.
Why Rapid COVID-19 Tests are “CRAPPY”.
P.P.P.S. If you want to help provide masks and gowns to our medical providers in NYC, then please donate to Bstrong initiative at Global Empowerment Mission.
P.P.P.P.S. I was informed today that Dasheng actually makes the “counterfeit” mask. My source told me that Dasheng makes both KN95 with earloops and the NIOSH mask. That this “counterfeit” might not be a counterfeit, but a mislabeling of a KN95 as a NIOSH by a major Chinese corporation. 4/16/2020
P.P.P.P.P.S. Thankfully, the Ministry of Commerce in China has recently started to crack down on its own export along with the guidelines that I have outlined. 4/19/20



