Counterfeit Respirators / Misrepresentation of NIOSH-Approval
SOURCE: https://medium.com/@jayparkmd/how-to-spot-a-fake-kn95-mask-from-china-d5219e7f0ab2
Counterfeit Respirators / Misrepresentation of NIOSH-Approval
Updated April 28, 2020
Counterfeit respirators are products that are falsely marketed and sold as being NIOSH-approved and may not be capable of providing appropriate respiratory protection to workers.
When NIOSH becomes aware of counterfeit respirators or those misrepresenting NIOSH approval on the market, we will post them here to alert users, purchasers, and manufacturers.
How to identify a NIOSH-approved respirator:
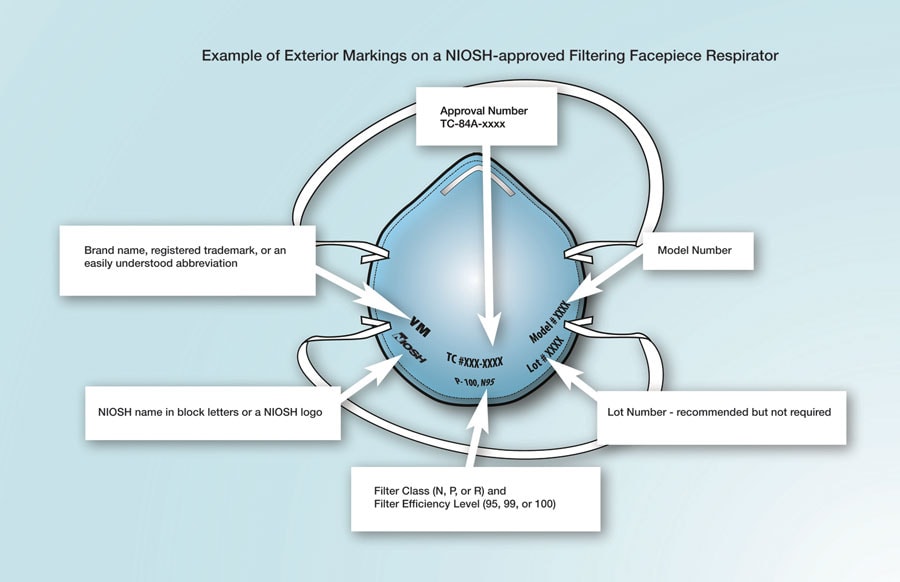
NIOSH-approved respirators have an approval label on or within the packaging of the respirator (i.e. on the box itself and/or within the users’ instructions). Additionally, an abbreviated approval is on the FFR itself. You can verify the approval number on the NIOSH Certified Equipment List (CEL) or the NIOSH Trusted-Source page to determine if the respirator has been approved by NIOSH. NIOSH-approved FFRs will always have one the following designations: N95, N99, N100, R95, R99, R100, P95, P99, P100.
Signs that a respirator may be counterfeit:
- No markings at all on the filtering facepiece respirator
- No approval (TC) number on filtering facepiece respirator or headband
- No NIOSH markings
- NIOSH spelled incorrectly
- Presence of decorative fabric or other decorative add-ons (e.g., sequins)
- Claims for the of approval for children (NIOSH does not approve any type of respiratory protection for children)
- Filtering facepiece respirator has ear loops instead of headbands
Additional Tips for Spotting Counterfeit Respirators Before You Buy

This is an example of a counterfeit respirator using Shanghai Dasheng Health Products Manufacture Co. Ltd’s (SDH) NIOSH approval number, TC 84A-4335, without their permission. SOUND is not a NIOSH approval holder or a private label holder. (4/28/2020)



NIOSH did not issue this letter and test report to Shenzhen Ende Medical Technology Co., Ltd. Although they appear to be from NIOSH, these documents have been altered and the information contained has been falsified. Shenzhen Ende Medical Technology Co., Ltd. is NOT a NIOSH approval holder. Any N95 filtering facepiece respirators from Shenzhen Ende claiming to be NIOSH-approved or accompanied by these documents are NOT NIOSH approved. (4/27/2020)
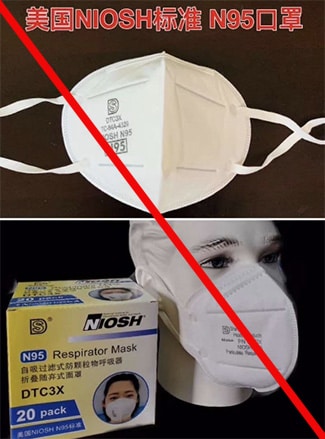

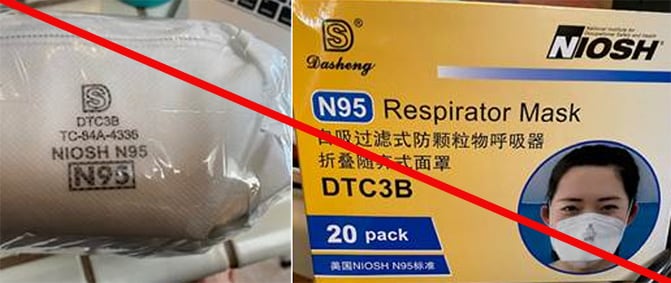


These are examples of counterfeit respirators using Shanghai Dasheng Health Products Manufacture Co. Ltd’s (SDH) NIOSH approval numbers without their permission. These models include, but may not be limited to, models DTC3X (marked as TC-84A-4329), DTC3W (marked as TC-84A-4335), DTC3B (marked as TC-84A-4336), DTC3Z (marked as TC-84A-8150), and Raxwell RX9501P. Note that any SDH respirators with ear loops are NOT NIOSH approved. (4/17/2020)

This is an example of a misrepresentation of a NIOSH-approval. G & F Products is not a NIOSH approval holder or a private label holder. (4/9/2020)

Any respirators being sold as Maskin are no longer NIOSH approved. They are counterfeit or they are no longer compliant to the NIOSH approval. (4/9/2020)
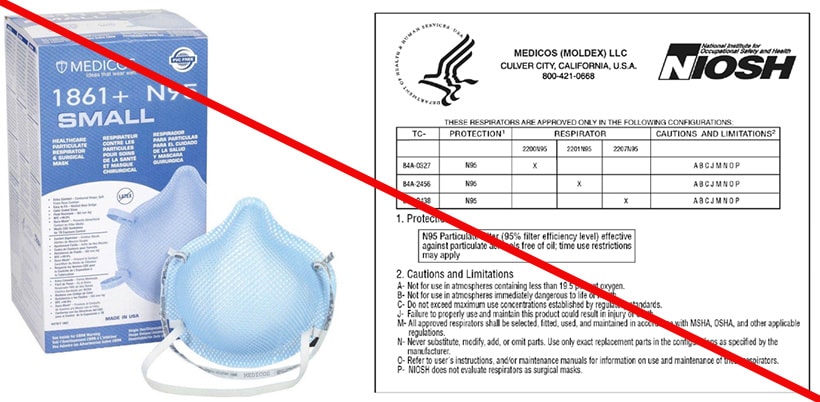
This is an example of a counterfeit respirator. Medicos is selling an N95 respirator using the Moldex approval number and label without Moldex’s permission. Medicos is not a NIOSH approval holder or private label holder. (3/12/2020)

This is an example of a misrepresentation of the NIOSH-approval. Yark is not a NIOSH approval holder or a private label holder. Additionally, respirators from the box include the CE (European) approval mark and NIOSH N95. This is not an acceptable format for a NIOSH-approved respirator. (3/5/2020)

The Guangzhou Weini Technology & Development Co., Ltd. (GWT) respirator with model number K320 is not NIOSH-approved. GWT respirator approvals were rescinded in 2009. Please refer to our user notice for additional information. GWT respirators bearing any NIOSH approval number listed on the user notice is NOT NIOSH-approved. (2/10/2020)

There are no markings on the face of the respirator. (11/6/2019)

NIOSH does not approve any type of respiratory protection for kids. (11/6/2019)

There are no markings on the face of the respirator. (11/6/2019)

This product is not NIOSH-approved. Look at the markings on the front. The logo is wrong, there is no approval number (TC-84A-xxxx). (11/6/2019)

This product is not NOSH approved. No NIOSH logo or approval number on the face of the product. (11/6/2019)

This product is not NOSH approved. No NIOSH logo or approval number on the face of the product. (11/6/2019)





















Images here are examples of counterfeit respirators. These respirators are being sold as if they are NIOSH-approved even though the manufacturer, Anhui Tongcheng YaGe Health Materials, Co., Ltd, is not listed as a NIOSH approval holder or a private label holder. (10/23/2019)
 |
 |
| These are examples of misrepresentation of the NIOSH-approval. PitBull Safety Products is not a NIOSH approval holder. (10/07/2019) | |

This is an example of misrepresentation of the NIOSH-approval. Vogmask® is not a NIOSH approval holder. This wording is misleading and not accurate: With premium technologies and designs for best particle filtering results, our NIOSH certified Vogmask® is a reusable superior everyday face mask that protects one from dust, fine particulate matters (PM), pollen, air pollution, such as smog and smoke … from https://www.vogmask.ca/external icon (10/07/19)

This is an example of two counterfeit respirators. Valpro Safety is selling the Ranger 821 and Ranger 821V respirators using the 3M approval number (TC-84A-007) and label without 3M’s permission. (6/19/19)

This is an example of a counterfeit respirator. Pacifico Salud SAC is selling units using the Suzhou Sanical Protection (SSP) approval number (TC-84A-6766) and label without SSP’s permission. Additionally, there are two errors on the respirator package. The first error is that they claim the N95 respirator is 96% efficient. The second error is located in the bottom right corner of the package where is states the respirator is manufactured by Benehal China, who is not a NIOSH approval holder. (1/4/2019)

This counterfeit respirator, NT-V2 Nano Bi-Directional respirator, is being advertised as a NIOSH-approved, using a NIOSH approval number. The TC number (TC 84A-0427) belongs to a 3M full facepiece respirator with cartridges and was used without 3M’s permission. Additionally, this counterfeit respirator was not manufactured by Pasture Pharma.
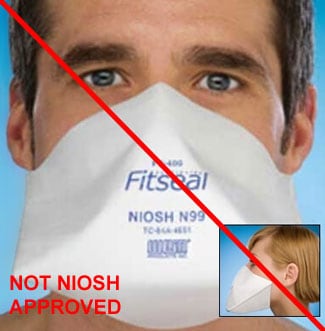
This respirator is being sold as if it is NIOSH-approved, even though the manufacturer, FitSeal, is not listed as a NIOSH approval holder or a private label holder. (2/19/2019)
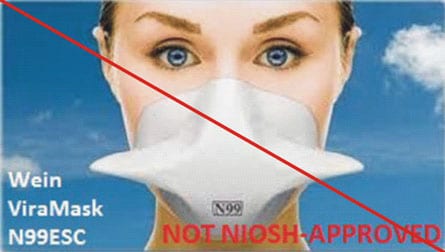
This is an example of misrepresentation of the NIOSH-approval. All approvals for Wein Products (WPI) were rescinded in 2011. However, the manufacturer’s website continues to state the ViraMask N99ESC is certified by NIOSH. View the user notice announcing the rescission.

This is an example of a counterfeit N95 Respirator that was brought to NIOSH’s attention. While the TC number and private label holder are valid, this unapproved unit can be identified by the misspelling of NIOSH on the front of the respirator.


These are examples of counterfeit respirators. These respirators are being sold as if they are NIOSH-approved even though the manufacturer, Zubi-Ola, is not listed as a NIOSH approval holder or a private label holder.
Check the respirator approval markings (graphic below) or the Certified Equipment List to verify your respirator is NIOSH-approved. Additional information is available on the NIOSH Trusted Source page.
Example of the Correct Exterior Markings on a NIOSH-Approved Filtering Facepiece Respirator